LAB #5 – Measurement: Mass, Volume, and the Exploration of Density
Introduction
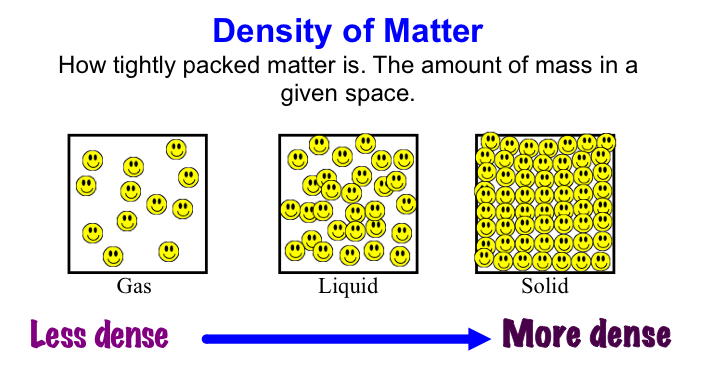
Volume is the amount of space occupied by matter - solid, liquid, or gas.
Volume is measured in units: cm3 for a solid, mL for a liquid.
Problem
- A: How can we find the volume of a rectangular block?
- B: How can we find the volume of an irregularly
shaped object?
Hypothesis:
Materials:
Various sized blocks, irregular shaped objects, ruler, graduated cylinder
Procedure A
1) Use a metric ruler to measure the dimensions of your rectangular objects; measure to the nearest tenth (0.1) cm.
2) Calculate the volume in cm3 of your rectangular object by multiplying the length (cm) times the width (cm) times the height (cm). V = L x W x H and round to the nearest tenth!
3) Record your measurements in the data table.
Results A
Data Table A: Volume of rectangular objects
Object
|
Length (cm)
z-axis
|
Width (cm)
x-axis
|
Height (cm)
y-axis
|
Volume
(cm3)
|
1)
|
|
|
|
|
2)
|
|
|
|
|
3)
|
|
|
|
|
4)
|
|
|
|
|
5)
|
|
|
|
|
V = L x W x H cm3 = (cm)(cm)(cm)
Procedure B
Use a graduated cylinder to measure the volume of an irregular shaped solid.
1. Fill the graduated cylinder to __ mL and record this into your notebook. This is your initial volume.
2. Carefully drop the object in on an angle. The object will displace water (push water up to make way for the object) which will rise to make a new volume.
3. Subtract your initial volume from the new water level.
Results B
1. Final Volume --------------à _____mL
- Initial Volume ------------à _____mL
=Volume of irregularly shaped object: _____mL
2. Final Volume --------------à _____mL
- Initial Volume ------------à _____mL
=Volume of irregularly shaped object: _____mL
Analysis B.1
1) What is the maximum volume you can measure with this graduated cylinder?
2) What is the smallest volume you can measure with this graduated cylinder?
3) Determine the value of the minor grids on the cylinder. i.e. how many mL does each line equal?
4) Now, check to see if you’ve measured correctly using the volume of a sphere equation:
a. Diameter of marble = ___cm
b. Radius of marble = ___cm (radius = diameter ÷ 2)
c. Calculator entry information:
4 ÷ 3 x π x radius x radius x radius
Volume of sphere using equation = ____cm3
5) Go back to Lab #2 – Crazy Coasters
Mass of glass marble: _____g
Volume of glass marble: _____cm3
Density of glass marble: _____g/cm3
Procedure C
The mass of an object is a measure of the number of atoms in it. The basic unit of measurement for mass is the gram (g).
You are going to calculate the densities of the wood blocks using the equation, Density = Mass/Volume. You already have the volume of wood blocks A, B, C, D, and E in the data table for Results A. You will use a triple beam balance to find the mass of each block, and then use the equation to find their volumes. As always, make sure to include the proper units and round to the nearest tenth.
Results C
Density = Mass/Volume
Object
|
Mass (g)
|
Volume (cm3)
|
Density
( g/cm3 )
|
1)
| | | |
2)
| | | |
3)
| | | |
4)
| | | |
5)
| | | |
Units:
Mass = grams = g
Volume = cubic cm = cm3
Density = ___ hint: D=M/V
Analysis B.2
5) How can you use a graduated cylinder to measure the volume of a liquid?
6) What happens to the volume of the liquid when you drop an object into the graduated cylinder? How can we use this to help us find the volume of the object?
7) If an object dropped into the graduated cylinder pushes up the water mark from an initial volume of 25.0 ml to a final volume of 51.5 ml, how many cm3 is the object?
8) Compare the two different methods of obtaining volume of a marble, how did you do? How far off were your calculations?
9) Go back to Lab #2 – Crazy Coasters
Mass of glass marble: _____
Volume of glass marble: _____
Density of glass marble: _____
++Include units++
Analysis A and C
1) Calculate: Combine the densities of blocks A, B, C, D, and E and find the average density for the wood. Show your work.
2) What is the maximum mass the triple beam balance can measure?
3) What is the minimum mass the triple beam balance can measure?
4) What are the units for the triple beam balance?
5) Why is it called a triple beam balance? What does each beam measure; think in numerical terms.
6) Why is it necessary to zero your triple beam balance before using it?
Determining the Density of Water
Procedure:
1. Measure the mass of an empty graduated cylinder, and record your data below.
2. Fill the graduated cylinder up to a certain volume, and record your data below.
3. Measure the mass of the graduated cylinder and the water, and record your data below.
4. Subtract the initial volume from the final volume to find the volume of the water.
Mass of graduated cylinder + water ______g
- Mass of graduated cylinder -______g
-------------------------------------------------------
Mass of water ______g
Volume of water = ___mL
Mass of water = ___g = Density of water___g/mL
Volume of water = ___mL
Analysis D
1) What is the density of water? Round to the nearest tenth and include units.
2) Compare/Contrast your Results with another group’s. How similar was the mass and volume? How similar was the density?
3) Density of water: _____g/mL
4) Density of wood: _____g/cm3
5) Density of glass: _____ g/cm3
6) Rank the above in order from least dense to most dense. Predict: If you were to drop the glass and wood into a tub of water, what would happen to the solid objects?
7) Sinking/Floating:
a. If you have a solid with a density less than 1.0 g/cm3 would it sink or float?
b. If you have a liquid with a density less than 1.0 g/cm3 would it sink or float?
c. If you have a solid with a density more than 1.0 g/cm3 would it sink or float?
d. If you have a liquid with a density more than 1.0 g/cm3 would it sink or float?
Conclusion
What was your problem?
|
Restate your hypothesis. Was it right? wrong? why or why not?
|
What did you learn in this lab?
|
What did you like about this lab?
|
What were some challenges you had to deal with?
|
What could you do next with this problem? What other tests could you perform?
|
Write down any other additional thoughts, observations, inferences, etc.
|